Close
Shop All:Innovating Science
Innovating Science Determination of The Empirical Formula of MGO AP CHEM
Item #:
2134313
$41.19 CAD
$37.07 CAD
This lab illustrates the (i) law of conservation of mass and (ii) the law of constant composition.
About This Item
Description
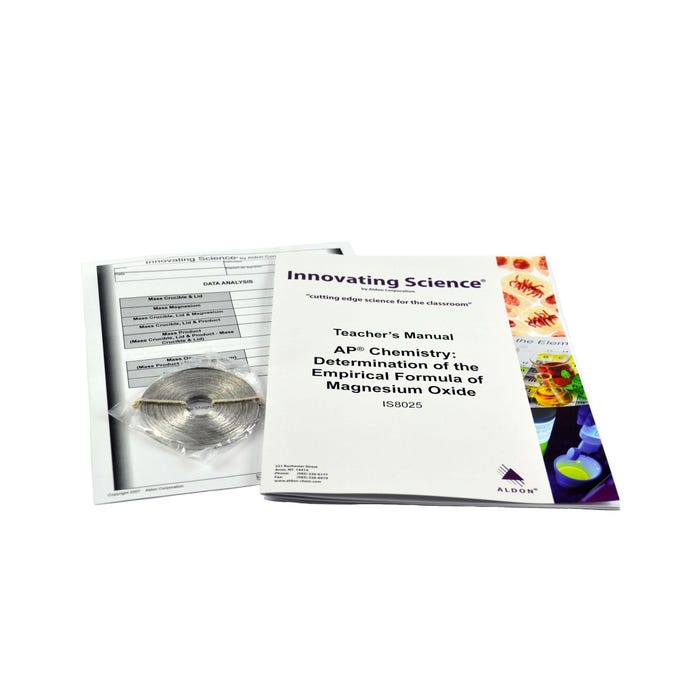
The quantitative stoichiometric relationships governing mass and amount will be studied using the combustion reaction of magnesium metal. Magnesium is reacted with oxygen from the air in a crucible, and the mass before and after the oxidation is measured. The resulting masses are used to calculate the experimental empirical formula of magnesium oxide, which is then compared to the theoretical empirical formula. A crucible and Bunsen burner will be used to heat magnesium metal to burning.Features
Includes:
-
1 Roll Magnesium Metal, Ribbon
Innovating Science Determination of The Empirical Formula of MGO AP CHEM